| Science > Chemistry > Periodic Table of Elements > Magnesium |
|
 |
|
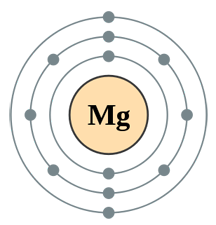
| Atomic Number of Magnesium | 12 |
| Atomic Symbol of Magnesium | Mg |
| Atomic Weight of Magnesium | 24.305 |
| Color of Magnesium | silver-white |
| Density of Magnesium | 1.738 g/cubic centimeter |
| Melting Point of Magnesium | 650 degrees C / 1202 degrees F |
| Boiling Point of Magnesium | 1090 degrees C / 1994 degrees F |
| Magnesium State At Room Temp | solid |
| Classification of Magnesium | metallic - alkaline earth metal |
| Date Magnesium Was Discovered | 1755 |
| Facts on Magnesium | Magnesium is an alkaline earth metal and highly reactive element. Magnesium is used in laxatives and milk of magnesia. Magnesium is used in flashbulbs, optical mirrows, fireworks, and incendiary bombs. Magnesium is the 8th most abundant element in the earth's crust and is the third most abundant element dissolved in seawater. The adult human daily requirement of magnesium is about .3 g / day. Magnesium is the 11th most abundant element by mass in the human body and mostly found within the bones. Magnesium is needed for more than 300 biochemical reactions in the body. Green vegetables such as spinach are good sources of magnesium because the center of the chlorophyll molecule (which gives green vegetables their color) contains magnesium. Eating a wide variety of legumes, nuts, whole grains, and vegetables will help you meet your daily dietary need for magnesium. Magnesium reacts with sulphur, nitrogen and the halogens. |
| Advertise | Contact Us | Top Sponsors | User Agreement |